by Hannah Chu, Ph.D. Student
Twitter: @hannahhchu
We were staring at a fuzzy photo of fly egg masses that resembled grains of rice thrown on a decaying body. “So, what do you think?” my advisor asked excitedly. As a student in one of the only forensic entomology research labs in New York City, I eagerly awaited new homicide cases that crossed my advisor’s desk. “Why don’t you and the others head down to the Office of Chief Medical Examiner (OCME) to collect samples?” my advisor communicated to me. Being a forensic entomology research lab, we used insect behavior and development to assist in legal investigations such as murders or animal abuse cases. Insects play a pivotal role in these investigations for forensic entomologists because many of them, most commonly blow flies (Family: Calliphoridae), can tell a lot about how long a body has been dead. Larval, or immature insect, developmental cycles help estimate the post-mortem interval (PMI), or the time since death; however, it is more accurately described as the minimum time of colonization (MTC). The MTC, defined as the time the first insect egg was laid, allows for a more accurate description of how forensic entomologists estimate the time of death. In our investigation, we combined a few common methods to narrow down the MTC including larval species identification and calculation accumulated degree days for development. I quickly ran to the lab and told my peers about the new case and we prepared to head to the OCME.
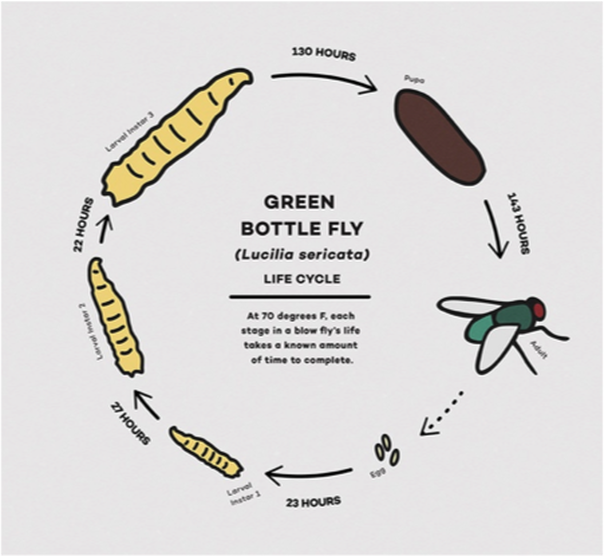
Once my lab mates and I made our way across to the city to the OCME, we followed an official-looking scientist into a small room, passing a few bodies covered in white sheets revealing only their feet. The scientist handed me a few vials of maggots and adult blow flies in ethanol. We carefully signed some chain-of-evidence forms and hurried back to the lab as we noticed one mistake made by on-scene investigators. The investigators did not boil the maggots to preserve their shape and morphological features. While it is possible to preserve maggots by just placing them in ethanol, long-term storage without boiling may damage defining features of species. Thus, once we returned to the lab, we drained the maggots and blanched them in boiling water. (My lab mate thought it smelled like steamed chicken…) Once the maggots were properly preserved, we had to determine the species and developmental stage of the maggots. To identify the developmental stage (first, second, or third instar), we observed the number of spiracles located on their posterior end (or better known as their butt, and yes maggots breathe out of their butt). Once we identified the developmental stage, we moved onto identifying the exact species of the collected specimens. Identifying the species becomes extremely important because each species develops at different rates, which can affect estimating an accurate PMI. Common fly families that colonize decaying animal tissue include Calliphoridae (blow flies), Muscidae (house flies), and Sarcophagidae (flesh flies) [1-3]. A typical life cycle of these flies described in the life cycle illustration occurs under controlled conditions; however, deaths usually occur in environments with varying temperatures, habitats, and other conditions that will largely affect larval development. Therefore, a professional forensic entomologist requires a deep understanding of insect metabolism and development in differing conditions, which is crucial to making accurate PMI/MTC estimations. As hopeful budding forensic entomologists, my lab mates and I cross-referenced the multiple species we identified based on morphological features and used their unique developmental timings to draw a conclusive estimation.
Twitter: @hannahhchu
We were staring at a fuzzy photo of fly egg masses that resembled grains of rice thrown on a decaying body. “So, what do you think?” my advisor asked excitedly. As a student in one of the only forensic entomology research labs in New York City, I eagerly awaited new homicide cases that crossed my advisor’s desk. “Why don’t you and the others head down to the Office of Chief Medical Examiner (OCME) to collect samples?” my advisor communicated to me. Being a forensic entomology research lab, we used insect behavior and development to assist in legal investigations such as murders or animal abuse cases. Insects play a pivotal role in these investigations for forensic entomologists because many of them, most commonly blow flies (Family: Calliphoridae), can tell a lot about how long a body has been dead. Larval, or immature insect, developmental cycles help estimate the post-mortem interval (PMI), or the time since death; however, it is more accurately described as the minimum time of colonization (MTC). The MTC, defined as the time the first insect egg was laid, allows for a more accurate description of how forensic entomologists estimate the time of death. In our investigation, we combined a few common methods to narrow down the MTC including larval species identification and calculation accumulated degree days for development. I quickly ran to the lab and told my peers about the new case and we prepared to head to the OCME.
Once my lab mates and I made our way across to the city to the OCME, we followed an official-looking scientist into a small room, passing a few bodies covered in white sheets revealing only their feet. The scientist handed me a few vials of maggots and adult blow flies in ethanol. We carefully signed some chain-of-evidence forms and hurried back to the lab as we noticed one mistake made by on-scene investigators. The investigators did not boil the maggots to preserve their shape and morphological features. While it is possible to preserve maggots by just placing them in ethanol, long-term storage without boiling may damage defining features of species. Thus, once we returned to the lab, we drained the maggots and blanched them in boiling water. (My lab mate thought it smelled like steamed chicken…) Once the maggots were properly preserved, we had to determine the species and developmental stage of the maggots. To identify the developmental stage (first, second, or third instar), we observed the number of spiracles located on their posterior end (or better known as their butt, and yes maggots breathe out of their butt). Once we identified the developmental stage, we moved onto identifying the exact species of the collected specimens. Identifying the species becomes extremely important because each species develops at different rates, which can affect estimating an accurate PMI. Common fly families that colonize decaying animal tissue include Calliphoridae (blow flies), Muscidae (house flies), and Sarcophagidae (flesh flies) [1-3]. A typical life cycle of these flies described in the life cycle illustration occurs under controlled conditions; however, deaths usually occur in environments with varying temperatures, habitats, and other conditions that will largely affect larval development. Therefore, a professional forensic entomologist requires a deep understanding of insect metabolism and development in differing conditions, which is crucial to making accurate PMI/MTC estimations. As hopeful budding forensic entomologists, my lab mates and I cross-referenced the multiple species we identified based on morphological features and used their unique developmental timings to draw a conclusive estimation.
In order to estimate the PMI, my lab mates and I calculated the accumulated degree days (ADD) of the different maggots. ADD is the official calculation used by forensic entomologists to determine the amount of time it takes for a maggot to reach each of its life stages based on how much heat (or temperature) has accumulated over each hour. Each species also has a development threshold, which when reached will stop developing. In simpler terms, the ADD is 24 hours multiplied by the temperature. Each species will have a specific ADD that corresponds to each of its developmental stages. However, you may be wondering how we could get the exact temperatures of the location a body was left. This makes things more complicated and often involved placing a data logger in the location after a body is found and back-calculating the temperatures of the location by comparing the data-logged temperature and the data from the nearest weather station. (I will not get into this because it gets quite complex, but if you’re curious, send me an email!)
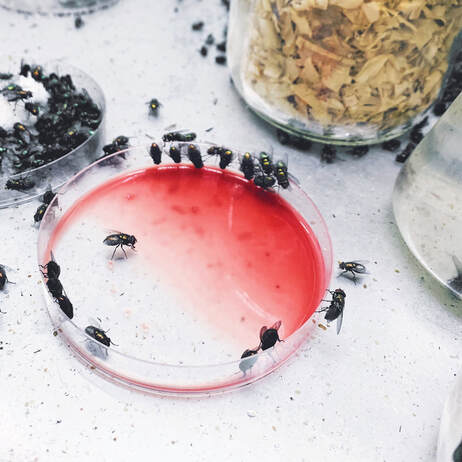
Third Instar Calliphora vicina maggots being reared in the lab.
After we identified the species, the developmental stages of the maggots, and the ADD of our specimens, we were able to combine all the data to come to our best estimate of the time the victim was killed. Our estimation matched our PI’s estimation which provided more support for our conclusion. This information was given to the lawyers and this was the end of my time as an investigator on this case. While working on this case, I walked away with a lot more questions about other lines of evidence that would really benefit forensic entomologists, such as general blow fly behaviors, interactions between species, and environmental stimuli. The field of forensic entomology has been understudied and neglected, which furthers its pseudoscience stigma. However, with the quickly developing molecular technologies, some labs have started using molecular tools to examine the genetic mechanisms that forensic entomologists can use to estimate even more accurate MTCs. Coming from a forensic background, I’m excited to see the advances that will be made in the field in the coming years.
References
- Byrd, J. (Ed.), Lord, W., Allen, J., Hawkes, R., Parker, M., Haskell, N., Anderson, G. (2001). Forensic Entomology. Boca Raton: CRC Press, https://doi.org/10.1201/9781420036947
- Amendt, J. et al. Best practice in forensic entomology--standards and guidelines. Int. J. Legal Med. 121, 90–104 (2007).
- Greenberg, B. (1991). Flies as forensic indicators. Journal of Medical Entomology, 28(5), 565–577.
- Braack, L. E. O. (1987). Community dynamics of carrion-attendant arthropods in tropical African woodland. Oecologia, 72(3), 402-409.
- Smith, K. G. (1986). A manual of forensic entomology.
- Rosati, J. Y. Spatial and Temporal Variability in the Carrion Insect Community: Using Blow Flies (Family: Calliphoridae) as a Model System to Study Coexistence Mechanisms at Multiple Scales.
- Ody, H., Bulling, M. T., & Barnes, K. M. (2017). Effects of environmental temperature on oviposition behavior in three blow fly species of forensic importance. Forensic Science International, 275, 138–143. https://doi.org/10.1016/j.forsciint.2017.03.001
- El-Moaty, Z. A., & Kheirallah, A. E. M. (2013). Developmental Variation of the Blow Fly Lucilia Sericata (meigen, 1826) (diptera: Calliphoridae) by Different Substrate Tissue Types. Journal of Asia-Pacific Entomology, 16(3), 297–300. https://doi.org/10.1016/j.aspen.2013.03.008
- Wall, R. (1993). The reproductive output of the blowfly Lucilia sericata. Journal of Insect Physiology, 39(9), 743–750. https://doi.org/10.1016/0022-1910(93)90049-W
- Thyssen, P., Souza, C., Shimamoto, P., Salewski, T., & Moretti, T. (2014). Rates of development of immatures of three species of Chrysomya (Diptera: Calliphoridae) reared in different types of animal tissues: implications for estimating the postmortem interval. Parasitology Research, 113(9), 3373–3380. https://doi.org/10.1007/s00436-014-4002-x


 RSS Feed
RSS Feed
